Matrisome of Ageing
Discussing the role of Extracellular Matrix components in the ageing process.
Extracellular_matrix (ECM) is a three-dimensional network consisting of extracellular macro molecules and minerals, such as collagen, enzymes, and glycoproteins that provide structural and biochemical support to surrounding cells and in formation of tissues. ECM characteristics and its features varies for young and aged cells and also for tissues during transduction signaling. Here, I tried to provide a snippet about mechano transduction sensors YAP/TAZ and its role in linking mechanosensing with ageing (particularly senescence). Lastly an overview of the Matrisome - An Inventory of Extracellular Matrix Constituents and Functions.
YAP1-LATS2 feedback loop dictates senescent or malignant cell fate to maintain tissue homeostasis
Dysfunction of the homeostasis-maintaining systems in specific cell types or tissues renders the organism susceptible to a range of diseases, including cancers. One of the emerging mechanisms for maintaining tissue homeostasis is cellular senescence. Authors reported that Hippo pathway plays a critical role in controlling the fate of ovarian cells. LATS2 and YAP1, two major components of the Hippo/YAP signaling pathway, form a negative feedback loop to control YAP1 activity and prevent ovarian cells from malignant transformation.
Understanding the role of YAP1-LATS2 feedback loop forming a homeostat for determining senescent or malignant fate using mechanistic modeling.
YAP/TEAD–Mediated Transcription Controls Cellular Senescence
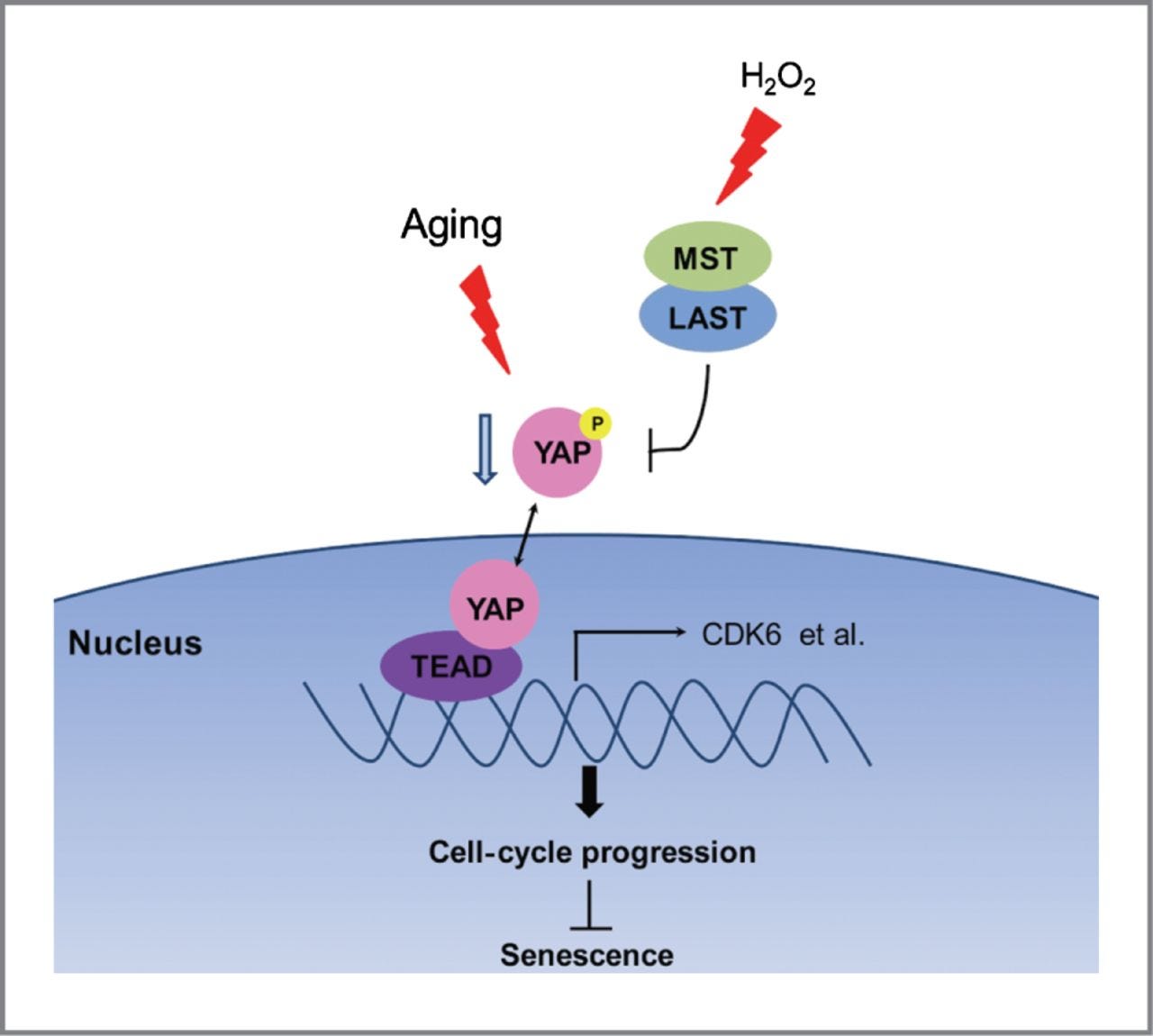
YAP–TEAD signaling plays an important role in the anti senescence through transcriptionally up regulating CDK6 expression. Replicative stress mediated downregulation of YAP and oxidation-mediated inhibitory phosphorylation of YAP lead to reduction of YAP–TEAD trans activation and promote cellular senescence.
The critical role of YAP in the regulation of cellular senescence provides a novel insight into a potential chemotherapeutic avenue for tumour suppression.
YAP/TAZ links mechanosensing to aging
During aging, extracellular matrix (ECM) integrity is decreased due to collagen fragmentation, glycation, crosslinking, and protein aggregation, leading to changes of physical, mechanical, and architectural properties of the ECM
YAP/TAZ plays an important role in preventing aging by preserving nuclear envelop (NE) integrity in the stromal cells. During aging, loss of nuclear YAP/TAZ due to reduced tissue mechanical force results in loss of NE integrity, which is followed by exposure of DNA to cytoplasm and cGAS-STING activation, leading to cellular senescence, and aging phenotypes.
YAP/TAZ activity in stromal cells prevents ageing by controlling cGAS–STING
A key challenge in ageing induced by cellular senescence is the identification of pathways that normally suppress senescence, are lost during ageing and are functionally relevant to oppose ageing. Authors connected the structural and functional decline of ageing tissues to reduced function of the master regulators of cellular mechano-sensing of YAP and TAZ.
YAP/TAZ activity declines during physiological ageing in stromal cells, and mimicking such decline through genetic inactivation of YAP/TAZ in these cells leads to accelerated ageing. Conversely, sustaining YAP function rejuvenates old cells and opposes the emergence of ageing-related traits associated with either physiological ageing or accelerated ageing triggered by a mechano-defective extracellular matrix. Ageing traits induced by inactivation of YAP/TAZ are preceded by induction of tissue senescence. YAP/TAZ mechanotransduction suppresses cGAS–STING signalling, to the extent that inhibition of STING prevents tissue senescence and premature ageing-related tissue degeneration after YAP/TAZ inactivation. Finding in the paper shows that decline in YAP/TAZ mechanotransduction drives ageing by unleashing cGAS–STING signalling, a pillar of innate immunity.
Sustaining YAP/TAZ mechanosignalling or inhibiting STING may represent promising approaches for limiting senescence-associated inflammation and improving healthy ageing.
Matrisome during Aging and Longevity
A Systems-Level Approach toward Defining Matreotypes Promoting Healthy Aging
Accumulation of damage is generally considered the cause of aging. Interventions that delay aging mobilize mechanisms that protect and repair cellular components. Consequently, research has been focused on studying the protective and homeostatic mechanisms within cells. However, in humans and other multicellular organisms, cells are surrounded by extracellular matrices (ECMs), which are important for tissue structure, function, and intercellular communication.
During aging, components of the ECM become damaged through fragmentation, glycation, crosslinking, and accumulation of protein aggregation, all of which contribute to age-related pathologies. Interestingly, placing senescent cells into a young ECM rejuvenates them. Furthermore, authors found that many longevity-assurances pathways reactivate de novo synthesis of ECM proteins during aging. This raises the question of what constitutes a young ECM to reverse aging or maintain health? In order to make inroads to answering this question, author suggest
A systems-level approach of quantifying the matrisome or ECM compositions reflecting health, pathology, or phenotype and propose a novel term, the "matreotype," to describe this.
The matreotype is defined as the composition and modification of ECM or matrisome proteins associated with or caused by a phenotype, such as longevity, or a distinct and acute physiological state, as observed during aging or disease. Every cell type produces its unique ECM. Intriguingly, cancer-cell types can even be identified based on their unique ECM composition. Thus, the matreotype reflects cellular identity and physiological status. Defined matreotypes could be used as biomarkers or prognostic factors for disease or health status during aging with potential relevance for personalized medicine.
Treatment with biologics that alter ECM-to-cell mechanotransduction might be a strategy to reverse age-associated pathologies. An understanding of how to reverse from an old to a young matreotype might point toward novel strategies to rejuvenate cells and help maintain tissue homeostasis to promote health during aging.
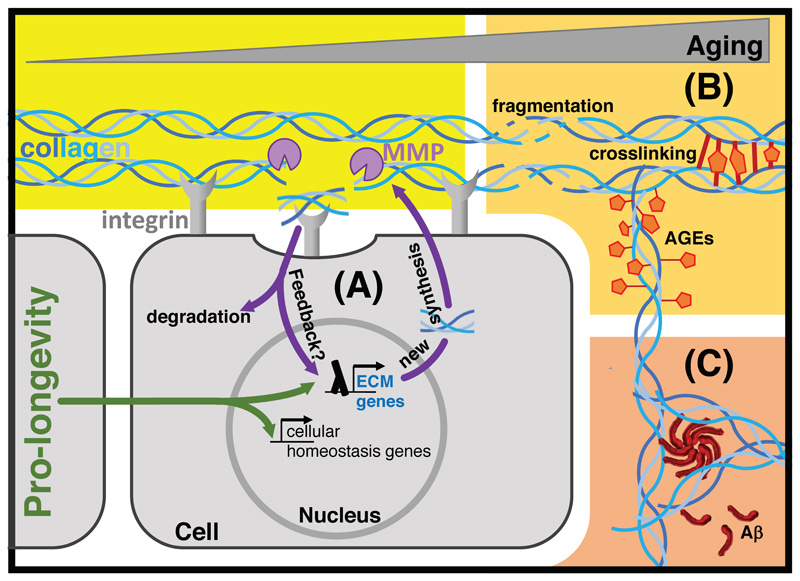
Hypothetical model of age-related damages to extracellular matrices
Pro-longevity interventions not only improve cellular protein homeostasis, but might also improve extracellular protein homeostasis.
(A) Cells synthesize and secrete proteins that form extracellular matrices (ECM). Cells are anchored to the ECM via cell-surface receptors, such as integrins. Integrins and other receptors transduce mechanical information via biochemical intracellular signaling cascades or cytoskeleton linkages to the nucleus. Matrix metalloproteases (MMP) cleave collagens and other proteins from the ECM.
(B) During aging, the ECM becomes fragmented, glycated, modified by advanced glycation end products (AGE), and/or crosslinked.
(C) Aggregation prone peptides, such as amyloid beta (Aβ), accumulate in extracellular matrices.
Ten Years of Extracellular Matrix Proteomics: “Matrisome”
The extracellular matrix (ECM) is a complex assembly of hundreds of proteins forming the architectural scaffold of multicellular organisms. Alterations of ECM composition, abundance, structure, or mechanics have been linked to diseases and disorders affecting all physiological systems, including fibrosis and cancer. Deciphering the protein composition of the ECM and how it changes in patho physiological contexts is thus the first step toward understanding the roles of the ECM in health and disease and toward the development of therapeutic strategies to correct disease-causing ECM alterations. Researchers have devised mass-spectrometry–based proteomic approaches to define the ECM composition, or “matrisome,” of tissues.
This review provides an overview of ECM proteomics, latest research, advances in profiling ECM of healthy and diseased tissues, identifying prognostic cancer biomarkers, challenges to translate finding to clinical application. Lastly, the review introduces readers to resources available to facilitate the interpretation of ECM proteomics datasets. In-depth exploration of the matrisome is within reach and we may soon witness the first translational application of ECM proteomics.
Resources for ECM Proteomics Research